Sarcoma diagnostic in a single reaction

Sarcoma Fusion Test
A unique test, based on a patented ligation-dependent PCR technology
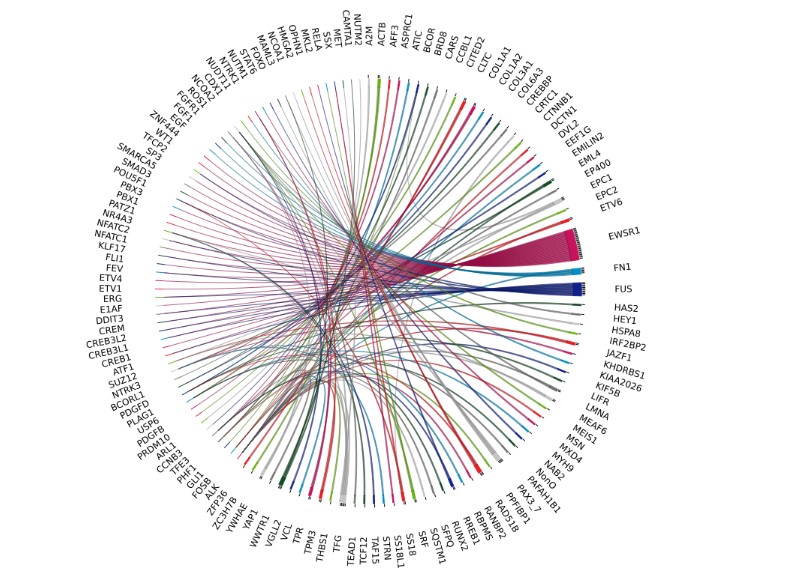
Fusion transcripts are frequently observed in around 1/3 of soft tissue tumours, and the scientific literature describes more than 140 different fusions.
These fusions are often associated with specific histological subtypes and targeted therapies, making them extremely useful molecular diagnostic markers.
Accurate detection of these fusion transcripts is of vital importance for optimal management of patients with sarcomas.
In this context, the SarcomaFusion test, developed by Genexpath, offers a cost-effective and efficient solution for detecting the fusion transcripts responsible for these sarcomas. Using this approach, sarcoma-specific fusion transcripts can be detected and assessed in a single comprehensive analysis, simplifying the diagnostic process and providing a better understanding of the molecular nature of sarcomatous tumours.
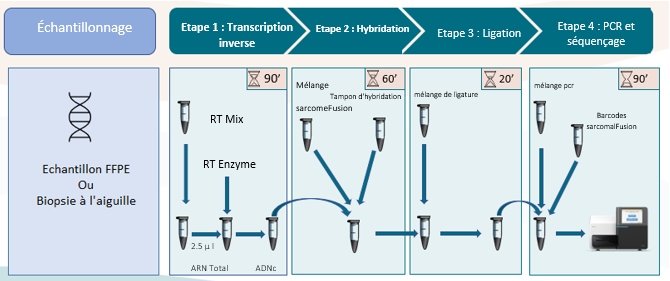
Use of the test
A quick and simple protocol
|
Precise computer analysis using dedicated software
Once sequencing has been completed, the FASTQ file can be uploaded to the RT-MIS platform. In just a few minutes of analysis, RT-MIS generates a file containing the fusion transcripts detected. RT-MIS also provides a bibliography associated with the transcripts detected, offering cancer researchers a complete solution.
| Handling duration | 5hrs30’ |
| Actual working time | 1hr-1hr30’ |
| Type of nucleic acid | RNA |
| Input quantity | Between 50 and 500ng of RNA in a volume of 2μl |
| Type of cancer | Sarcoma |
| Contents of the reagent kit | Probes targeting more than 140 fusion transcripts, barcodes, sequence primers |
| Method | Ligation dependent RT-PCR |
| Description | Detects more than 140 transcripts of sarcoma-associated fusion in 1 single analysis. |
| Equipment compatibility | MiSeq, NextSeq 500, NextSeq 550 Illumina® |
| Type of samples | Tissue biopsies fixed and included in paraffin |
| Technology | Next Generation Sequencing |
RT-MIS is therefore simple, fast and secure for the user.
Now, you can Purchase the Kit that matches your needs and be sure to obtain the best results in a short of time
| Products | Size | Cat# |
| SarcomaFusion Test |
8 reactions |
GEP-SF8 |
| 16 reactions |
GEP-SF16 |
|
| 24 reactions |
GEP-SF24 |
|
| 48 reactions |
GEP-SF48 |
For More information :